
“Since we started BioGaia 32 years ago, we have developed into a world-leading probiotics company at the forefront of microbiome research with safe, effective and consumer-friendly products on the global market. This is a very strong platform that we are now leveraging to become even more consumer-oriented.”
Key figures 2021
Key events 2021 - Q1
- BioGaia establishes BioGaia Invest, a new subsidiary focusing on innovative investments
- MetaboGen secures ethical approval to start new clinical study for prediabetes
- BioGaia revises dividend policy
- BioGaia launches entire Adult Health portfolio in Sweden
Company presentation
Become the world's most trusted global probiotics brand.
Food supplements with clinically tested, high-quality probiotics that are sold through pharmacies and via online retail platforms.
BioGaia’s share is listed on the Mid Cap segment of the Nasdaq OMX Nordic Stockholm Exchange.
To provide the world with probiotics that have clinically proven positive effects on health and well-being.
Innovation / Collaboration / Passion


BioGaia’s strong research core crucial for long-term profitability
BioGaia becomes even more consumer-oriented
The digitalisation trend, which was given an enormous boost in 2020 due to the Covid-19 pandemic, continued at the same pace in 2021. We took a number of important steps on our strategic journey to move closer to consumers and noted a highly positive trend, particularly in the USA, China, Vietnam and Indonesia, where we use an omnichannel strategy and e-commerce dominates the market. We are yet to fully recover in Europe, where digital developments are progressing slightly slower and sales are primarily through pharmacies.
During the year, we continued to build the organisation to complement our already strong position in business-to-business with a strong presence also in business-to-consumer. Our goal is to meet consumers on platforms that they use in all of our markets and to continue to invest in research and development in order to solve health problems for people all over the world. The right skills and change leadership are crucial in this transition. Several central new functions are now in place and work is continuing to integrate these into the existing organisation.
Unique strengths compared with our competitors
In the fierce competitive environment, with many large and active players in the market, BioGaia possesses unique strengths lacking in several of our competitors. We conduct our own research and development and use clinical studies to show that the products we make are really effective. Research is in our DNA and is something we will never sacrifice. Our research is indication-directed. This means we start with a condition such as colic, constipation and osteopenia and conduct pre-clinical and clinical studies that focus on this particular condition. Few other companies use this approach.
During the year, BioGaia Pharma received formal approval from competent authorities to proceed with both of its planned clinical studies. It is fantastic that we could successfully start two clinical trials of a potential pharmaceutical despite limited resources and are now progressing to the next phase in drug development.
We are also constantly striving to remain at the fore-front in respect of new product formulas. BioGaia was first in the world to launch drops and chewable tablets. We want our products to be convenient for consumers and to work in everyday situations and to always be based on the insights we gain from our consumer surveys.
Strong platform for profitable growth
BioGaia’s brand is already global and we have a better spread than all of our competitors, largely thanks to our outstanding distributors who work hard to get doctors, dentists, midwives and nurses to understand and recommend our products to consumers. Our broad spread is an excellent tool in the fierce competition for consumer attention. Our global presence is also a very strong platform that we can leverage on our journey to become even more consumer-oriented. Combined with our clinically tested products that have been proven to be effective, this will provide the conditions needed for long-term growth and profitability in the major shift currently taking place in the market due to digitalisation.
Chairman of the Board of BioGaia

Continued focus on building the world’s most trusted probiotics brand
Greater presence in key markets
Overall, 2021 was a good year when many important pieces fell into place in our efforts to continue to build BioGaia’s brand. I am pleased by the professionalism shown by the organisation in delivering on its commitments. Despite continued sluggish sales in Europe, this was offset by the strong performance in other parts of the world. In Asia, sales increased in part due to our online ventures in China, our sales via dental surgeries and our own e-commerce platform in Japan as well as advantageous demographic trends in Indonesia and Vietnam.
The USA accounted for the most outstanding development, where several successful launches of new products together with our omnichannel strategy yielded another record year. The USA is now BioGaia’s largest market and it was a great pleasure to sign an agreement at the end of the year to acquire our American distribution partner Everidis. BioGaia and Everidis have been partners since 2007, where Everidis has leveraged its unique understanding of the USA probiotics market and been highly successful in its omnichannel strategy combined with marketing to the healthcare sector and end consumers. This approach has set a standard for the whole BioGaia group. I am now extremely pleased that we have made this partnership permanent, can contribute resources, strengthen the pace of efforts to reach out directly to consumers, and build BioGaia into a leading brand in the USA.
We are currently present in 110 countries, through distribution partners and our own subsidiaries. We established BioGaia Japan in 2006, with a unique concept targetting dental clinics. At the beginning of the year, Protectis Mum was launched for pregnant women with very good results while we concurrently increased our presence in digital channels.
In Sweden, we took over from the previous distributor of the Adult Health portfolio. BioGaia is now available at the main pharmacy chains and on e-commerce websites, online pharmacies, from Amazon and in our own online store and we have continued to grow in all channels compared with last year.
We have also taken over distribution in Finland, where BioGaia’s products are now available at Finnish pharmacies and e-pharmacies under our own brand. In November, we started our own sales company in the UK. Our products will now be sold via our own online store, from Amazon and at selected pharmacy chains both online and offline.
New products and uniform brand offering
As a means of achieving our target to meet consumers regardless of where they buy their probiotics, we must invest even more in marketing directly to consumers and we intensified our efforts to add more consumer-oriented communication during the year. We have strengthened the management team with a new function, BioGaia Digital, which is similar to a digital incubator. BioGaia Digital works closely together with marketing and sales to support the organisation and our partners with guidelines, tools and content to ensure a uniform brand offering.
At the same time as we are investing in both physical and digital sales channels, we are also striving to develop new products together with our amazing distribution partners. Consumers are increasingly aware and want to know what the products contain, and quality and safety are crucial elements. In this respect, BioGaia has a unique strength and competitive advantage since research and clinical studies form the foundation of our offering. Our origins are in developing clinically tested probiotics of highest quality for children and we are now evolving to focus on great products for consumers throughout their life cycles. We want to improve global health, which is also one the UN Sustainable Development Goals. It is very gratifying that our research company has made tremendous progress during the year. BioGaia Pharma has received approval for two clinical studies and MetaboGen is focusing on developing next-generation probiotics.
Stepping up sustainability work
We have continued to work hard with sustainability and have taken several steps towards the goal of integrating it in all parts of the business. One highly positive effect of our strategy to increase our direct presence in markets has been that we now have greater influence over our environmental impact. We have stepped up efforts to reduce emissions from our packaging and to phase out palm oil, introduced a whistleblower system, held Group-wide anti-corruption training programmes, implemented a Code of Conduct and increased diversity in both management and on the Board. In addition, we continued to pursue our strong and important community engagement by supporting various organisations in health-promoting activities and through the Prevent Antibiotic Resistance (PAR) foundation. As a result of our hard work, in November we welcomed the fact that MSCI raised BioGaia’s ESG rating from BBB to A, an important endorsement for us and our shareholders.
There is clear and strong growth in the health trend and in preventive healthcare. People are increasingly interested in proactively improving their health, and probiotics can help to provide a balanced intestinal flora and an effective immune system. Given BioGaia’s passion, inherent potential, strategy and cooperation, I am very optimistic about the future. I would like to take this opportunity to thank all of the exceptional and dedicated employees at BioGaia, our customers and other partners for a marvellous job this year. Together, we have the necessary prerequisites to achieve our vision to become the most trusted probiotics brand.

CEO BioGaia
BioGaia's subsidiaries

MetaboGen
“I look forward to yet another exciting year with MetaboGen, where we take decisive steps forward in 2022 towards the launch of our first product.”
Sara Malcus, Managing Director MetaboGenMetaboGen
MetaboGen is a wholly owned subsidiary that conducts research and product development focusing on the microbiome and its potential to become the next generation of probiotic products.
During 2021, MetaboGen has achieved several important milestones in our development projects. In addition, we have continued to create the right conditions for our own product development, with new premises, collaborations and new employees.
MetaboGen has developed a unique production technology that helps oxygen-sensitive bacteria survive in a product. Such bacteria exist in healthy intestinal flora, but are lacking when the balance of the flora is disrupted. They therefore have great potential as new probiotic products. In 2021, MetaboGen’s unique technology received patent approval in most countries.
After a long wait due to the pandemic, we could finally begin META003 in August 2021, a clinical study in which we are studying the effect of our first product on individuals with an elevated risk of developing diabetes. The study, which is taking place at the Sahlgrenska University Hospital in Gothenburg, is fully engaged in enrolling participants and is expected to continue throughout 2022.
In 2021, we concluded an exploratory study of 100 pregnant women with Intrahepatic Cholestasis of Pregnancy (ICP), in partnership with Ferring Pharmaceuticals. The study was carried out at Kvinnokliniken in Lund and Södersjukhuset in Stockholm. Ferring ultimately chose not to continue with the project, though the results of the study were interesting and we now have an opportunity to find other licensees, or alternatively develop a product ourselves.
Over the past year, we also concluded the first phase of our project in melanoma indications, where the micro-biome appears to influence the outcome in connection with immunotherapy. We can see a potential for our strains in the field and are currently investigating the prospects for clinical studies and continued development.
Over the past year, MetaboGen has expanded its organisation with new employees and continued work with product optimisation and moved to new, larger premises with specialised laboratories. Today, we have very good capacity to manage the entire development chain, from the identification of new bacteria strains, through safety assessment and product development to the design and implementation of clinical studies. We are also continuing to develop our fermentation capacity on a small scale and currently manufacture, together with the fellow subsidiary BioGaia Production in Eslöv, products for clinical studies, in oxygen-free conditions.

BioGaia Production
“The year has been full of various challenges due to the pandemic. We successfully maintained production levels and deliveries according to plan without any delays to customers.”
Peter Persson, Managing Director BioGaia ProductionBioGaia Production
BioGaia Production AB is a wholly owned subsidiary of BioGaia AB that manufactures and packages BioGaia’s drops in glass bottles and plastic tubes, tablets/capsules in bottles, minipacks and product samples. BioGaia Production AB has a Good Manufacturing Practice (GMP) certificate from the Swedish Medical Products Agency.
BioGaia Production AB is a critical facility in BioGaia’s operations and, thanks to strong and coordinated efforts from all employees, we successfully maintained production levels without any delays to customers.
In 2021, we continued our ongoing efforts to streamline operations and experienced strong growth and increased sales without the need to employ more staff. Our operations are particularly vulnerable as they require people to be on-site or production would cease. It requires everyone to roll up their sleeves and help out when unforeseen events occurred. This has been our everyday life for the past two years and the whole organisation has acted in this way.
During the year, we received another GMP certificate, drug licensing authorisation to produce and package study products that are to be sent to the two clinical studies beginning in 2022. We also began constructing production premises for a pilot plant that will consist of a 100-litre fermentation facility (the expansion is progressing as planned and the municipality is expected to receive approval for the new development plan during the first quarter of 2022).
The year has been full of various difficulties due to the pandemic, relating to everything from the delivery of material and raw materials to having healthy personnel on site. I am very proud of all of the loyal, diligent and helpful employees who always do their utmost and support and help each other across all boundaries. Without them, we would never have seen the outstanding performance and growth we are experiencing today. We are ready to face the challenges that 2022 can bring.

BioGaia Pharma
“We are delighted this year to have reached our ambitious goal to start clinical trials in both of our development programmes.”
Nigel Titford, Managing Director BioGaia PharmaBioGaia Pharma
BioGaia Pharma applies extensive experience in probiotics research and development in the develop-ment of new biotherapeutic product platforms. BioGaia Pharma selects and develops pharmaceutical candidates based on supporting pre-clinical and clinical research.
In 2021, BioGaia Pharma took several steps towards building support for its prioritised programmes, which aim to supply effective pharmaceuticals based on live bacteria where a medical need exists and where the current standard of care is not optimal.
2021 was mainly marked by preparations to start clinical studies for the two pharmaceutical candidates in our two prioritised programmes. During the summer, we received formal approval from competent authorities in France and Sweden to move to the next phase. Before the end of 2021, the study product was filled and packaged (by our product partner BioGaia Production) and is now available for all of the six centres involved in the studies. Enrollment of patients also began at the end of 2021.
The first programme, BGP014, concerns Ulcerous Colic (UC). Ulcerous Colic is a debilitating condition characterised by chronic inflammation of the intestinal tract. The first stage of this programme is to study safety, tolerance and preliminary efficacy of BGP014 in the approved Phase I trial at several centres on UC patients with mild to moderate symptoms. The trial is taking place in Sweden.
The second programme, BGP345A, aims to treat constipation in patients receiving opioids to relieve pain. The study is a Phase II trial at several centres to assess the safety and preliminary effects. The study is taking place in France.
Looking ahead, we are also preparing for the next stage of enrolling patients and completing the clinical trials and look forward to the next and future phases.

BioGaia Invest
“We look forward to new opportunities in 2022 and to the ongoing collaboration with Boneprox and Skinome, which we are convinced will lead to increased growth for BioGaia.”
Sebastian Schröder, Managing Director BioGaia InvestBioGaia Invest
Since 2021, BioGaia Invest is a new wholly owned subsidiary of BioGaia AB that focuses on identifying and investing in small companies concentrating on breakthrough technology, services and products that can promote BioGaia’s growth.
BioGaia AB established BioGaia Invest in March 2021 to drive strong growth and remain at the forefront of research and innovation. During its first year of operation, the BioGaia Invest invested in Boneprox and Skinome, which are experts in osteopenia and skincare products that strengthen the skins natural microbiome, respectively.
With the investments, we can create new communication and information channels that will help BioGaia to leverage new technology and innovations.
Boneprox is a company specialising in artificial intelligence in dentistry, with a focus on linking dentistry with healthcare. Boneprox patented technology can be used to detect osteopenia using ordinary dental x-rays. Osteopenia is a disease characterized by low bone density, which leads to a fragile skeleton. Early diagnosis is crucial to prevent the disease from becoming a great burden to the patient and the healthcare system. In conjunction with the investment, Boneprox and BioGaia Japan will cooperate to detect patients at risk of osteopenia, while in parallel BioGaia’s bone health product, BioGaia Osfortis, will be offered to individuals with low bone density.
The second investment was in Skinome, a company that works with “microbiome-friendly” skincare. Skinome’s products are developed to strengthen the skin’s natural microbiome. BioGaia and Skinome will collaborate in the development of microbiome-friendly probiotic skincare products, both for Skinome and for roll out in BioGaia’s international network.

Product portfolio for the health of the whole family
The Paediatrics segment currently accounts for nearly 80% of BioGaia’s total sales. The vision for the product portfolio in mother and child is to launch products specifically for mother’s health and to create a broader portfolio in child health using new probiotic strains that enable us to target new indications. The portfolio has evolved from containing more broadly based products to more specific products.
Product launches in 2021
BioGaia Prodentis Kids
Even if most parents do their best to ensure that children brush their teeth regularly, there is still a risk that children develop caries. With varying genetic susceptibility and diet, everyone has different degrees of resistance to the pathogenic bacteria that are the root cause of caries. In 2021, BioGaia launched Prodentis Kids, which can help to maintain a healthy balance in the mouth, and reduce risk factors in the development of caries and inflammation of the gums.
The product contains a combination of probiotics and Xylitol. Probiotics are not only important for the gut, but also for health and balance in the oral microbiota. Xylitol helps to maintain the mineralisation of teeth. The patented probiotic strains, L. reuteri DSM 17938 L. reuteri ATCC PTA 5289, which are present in the product, act synergistically to improve oral health. The product can be used as a complement to brushing your teeth every day.
BioGaia Prodentis Kids has been tested in clinical studies and is safe for children to use.
BioGaia Protectis Immune Boost
Interest in preventive treatment and products that can strengthen the immune system has grown during the pandemic1). In 2021, BioGaia launched the BioGaia Protectis Immune Boost product, which contains a combination of probiotics and vitamin D and has demonstrated clinically-proven effect in a number of studies. BioGaia Protectis Immune Boost is a series of products suitable for the entire family.
Since up to 80% of the immune system is present in the gut, a healthy microbiota is important for a well-functioning immune system. BioGaia’s probiotic strain L. reuteri DSM 17938 has immunomodulating effects. It can prevent pathogenic bacteria from multiplying in the intestines. Combined with the immunosupportive vitamin D, the product promotes an improvement in general health.
BioGaia Protectis Immune Boost is available as oil drops, chewable tablets and capsules with different levels of vitamin D suitable for all ages.
Biogaia Pharax
The common cold and sore throat are the most common infections in the upper respiratory tract in children of preschool age, and are a significant reason for visiting a paediatrician and for absence from school and work. Pharax is a new product from BioGaia that aims to strengthen the oral immune system. The product has been found to reduce the duration and severity of upper respiratory tract infections in children.
BioGaia created Pharax to offer a probiotic complement to normal analgesics and as an alternative to antibiotics, which have no effect on virus infections. Given that Pharax is a natural and non-invasive product, parents can give Pharax to their children without the worry of adverse side effects.
Pharax probiotic drops are safe to use for children, and are free from milk protein, gluten and lactose. They have no side effects, and only supply healthy and positive bacteria with antiviral properties. Five drops given twice a day (morning and evening) for ten days have been shown in studies to reduce the duration and severity of a cold. Suitable for children from six months up to the age of five.
70% of colds are caused by viruses. The common cold is the most common disease among children up to the age of five and the main reason for visiting a paediatrician. A child of preschool age has, for example, on average six to eight colds per year, and the spread of infection is greatest in families with children that attend preschools. Most symptoms are mild; runny and blocked nose, sneezing, slight fever, sore throat and cough.
Antibiotics are only effective in diseases caused by bacteria and most common colds are caused by viruses, which leave parents with few alternatives apart from analgesics to offer some relief to their children.

Trends in society
Increased illness and growing health interest
Insights gained from a market survey completed in May 2021 indicate that consumers have become increasingly concerned about their basic health, such as vulnerability to diseases and ability to recover from sickness. The prioritisation of, and interest in, health with a focus on the immune system has increased around the world. 64 % of the global consumers in the survey stated that they have take action to improve their health and immune system over the past year (2020). 70 % of the polled have taken active measures to strengthen their health and immune system, compared with 53 % who answered the same question one year earlier.
The trend whereby a growing number of consumers want to reduce the risk of falling sick and retain good health confirms a change in consumer behaviour and that BioGaia is on the right path by adding consumer communication in channels complementary to the healthcare system. Previously, BioGaia mainly came into contact with consumers first when they sought medical care and needed treatment. As a large consumer group is thinking more about preventing disease and maintaining good health, it is important and relevant for BioGaia to catch the attention of consumers at an earlier stage, on Google, via PR articles, advertising on health website or via relevant influencers.
In the same survey and when answering questions about the health areas they hope to improve in the coming year, gut health and the immune system were the most popular areas and are areas in which BioGaia offers health-promoting products.
Changes to consumer purchasing behaviour
Digitalisation and the transition to online purchasing, which has been a clear trend for many years, have accelerated as a result of the pandemic. The lockdown of society and restrictions on physical contact have even convinced parts of the population that were previously reluctant to use technology to choose digital alternatives.
With the continued restrictions in society in 2021, the change in consumer purchasing behaviour, which was identified during the first year of the pandemic, has continued). E-commerce has grown and a broader target group is now opting to search for information and shop online. Food and health products are goods that have noted a clear upturn in e-commerce). In line with its omnichannel strategy, BioGaia has intensified work to add communication directly to consumers in digital channels and make its products available via e-commerce.
Lifestyle and preventive healthcare
The world’s population is growing and ageing, and as a result there is increasing interest in health. The western lifestyle has spread across the world in recent decades, as have the resulting lifestyle diseases. The physical and mental health of a growing number of people are being impacted by stress, sedentarism and poor diet. Concurrently, a rising number of consumers are taking greater responsibility for their health by seeking information about healthy diets and preventive healthcare. The interest in health products and food supplements is therefore expected to continue to rise sharply. Interest has grown in recent years in the importance of intestinal flora for our health and it is increasingly clear that the intestinal flora changes as we age.
Trends in the wider world provide both challenges and opportunities for BioGaia and our ability to create value for stakeholders through our research and our products.
Unrelenting growth in antibiotic resistance
The focus on the pandemic has overshadowed another pending global disaster, galloping antibiotic resistance. According to field reports and surveys from the WHO, there is a risk for further spread of antibiotic resistance following an increase in the incorrect over-use of antibiotics due to the pandemic.
With their immuno-strengthening effects, probiotics may have an important role to play in the fight against antibiotic resistance. A study published in the European Journal of Public Health shows, for example, a link between intake of BioGaia’s probiotic L. reuteri DSM 17 938and a reduced need for antibiotics in children.
The Prevent Antibiotic Resistance (PAR) foundation, which BioGaia started in 2017, makes a financial contribution to research and information on measures to reduce the need for antibiotics. In 2021, funds were distributed to projects focusing on preventive measures among elderly individuals.
Increased focus on sustainability
Few people can have missed the COP26 climate summit and the major humanitarian crisis cause by the pandemic worldwide. Consumers, and also other stakeholders, have become increasingly aware and sustainability is more frequently an important demand imposed in connection with purchasing or collaboration. Issues related to responsibility, environmental impact, business ethics and control are more important from a business and risk perspective. Customers want sustainable products and services while suppliers want to work with innovative, long-term and stable customers. Acting responsibly is also more highly valued by the employees of today and tomorrow.
In 2021, BioGaia took additional steps in the right direction to ensure good business ethics, improve its environmental impact and offer a workplace that motivates and creates well-being. Read more on page 36.
2 E-barometern; Statista 2022 Global number of digital buyers 2014-2021
3 McKinsey & Company Covid-19 US Consumer Pulse Survey 6/15-6/21/2020; E-barometern
Value creation for
BioGaia's stakeholders
- SocietyBioGaia contributes to society by being a good citizen and by offering products that contribute to improved health.
- DistributorsBioGaia builds long-term relationships with distributors and supports them within sales and marketing.
- ConsumersBioGaia offers consumers probiotic products that contribute to improved health.
- SuppliersBioGaia contributes to development of suppliers through long-term relationships and being a reliable partner.
- EmployeesBioGaia offers its employees a meaningful and stimulating workplace where employees feel job satisfaction.
- ShareholdersThrough good risk management and a long- term strategy BioGaia offers a stable value appreciation for its shareholders.
- ResearchersBioGaia contributes to increased knowledge of probiotics.
to society for ill health.
- SEK56.4min tax
- 167employees
- SEK3.6min community engagement
- 37new products launched during the year
- 13%of net sales to research and development
- 8%average growth in past 5 years
- 236published articles (Dec 2021)
- 32%operating margin
- 20,000individuals have participated in clinical studies (Dec 2021)
- 100%of tablet product range available as palm oil-free variants
BioGaia's value chain
- BioGaia conducts its own research as well as collaborating with a large external researcher network. Research includes everything from developing and improving methods for how probiotic cultures are produced to extensive pre-clinical and clinical research.
BioGaia also works strategically to identify new bacteria strains for the probiotics of the future.


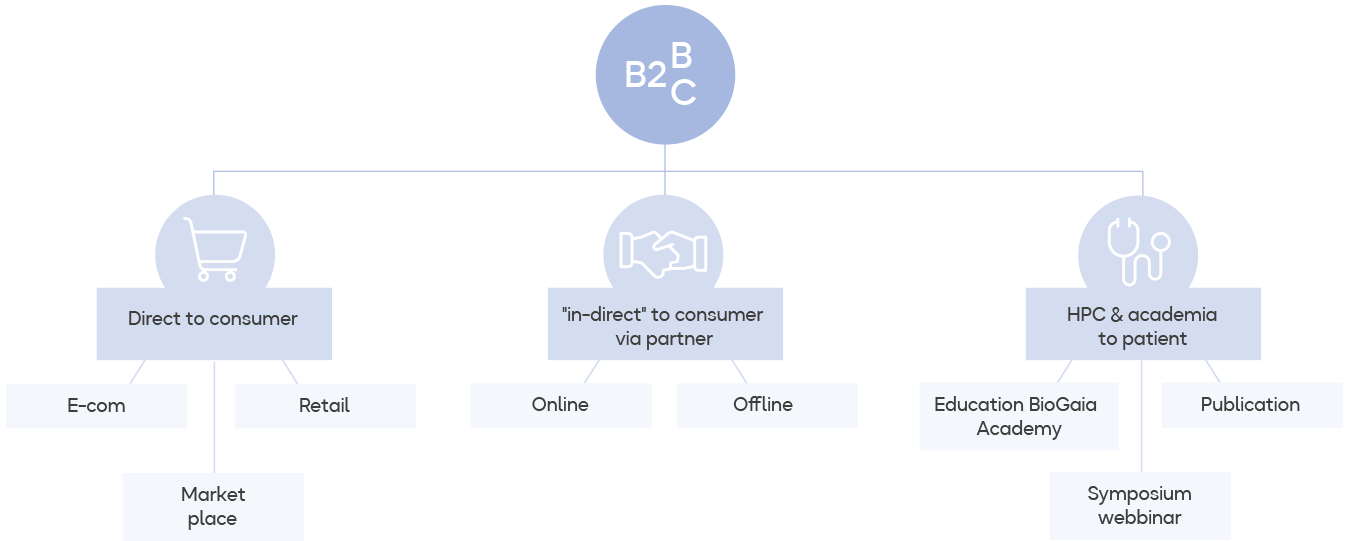
Omnichannel strategy the way forward
Over the past 32 years, BioGaia has built a top-class medical marketing model. By focusing on solid research, BioGaia shows doctors that the products are effective and doctors therefore then recommend the products to their patients. This still forms the basis of marketing and will continue to do so, but consumers’ buying behaviour and customer journey have changed in pace with digitalisation and new social channels. In 2020, the pandemic led to a rapid acceleration in e-commerce by 40%, an increase that continued at the same rate in 2021.
“If we are to remain relevant and raise awareness of probiotics in an increasingly competitive market, we must be available where consumers are, understand their needs and talk their language. We want to be part of the entire consumer lifecycle. We are already very good at medical marketing and have added expertise during the year in marketing directly to consumers,” says Linda Hägglund, Vice President Marketing.
The markets in which BioGaia operates are at different levels when it comes to digitalisation. There is also some variation depending on whether BioGaia is present itself in the country or distributes the products through partners. The first stage in building a strong global brand is to draw up new visual guidelines, to update the brand platform and to create tool kits that BioGaia’s partners and own subsidiaries can use in marketing to consumers.
Ultimately, the aim is to be relevant for consumers regardless of which channel or stage in the customer journey. This expansion in marketing requires more ability and capacity in several areas and the organisation was strengthened during the year with a new function, BioGaia Digital, led by Sebastian Heimfors.
BioGaia Digital is similar to a digital incubator and works closely together with marketing and sales to build a strong, clear and uniform brand offering in all digital channels.
“The entire retail landscape is in the midst of a transformation to follow a customer journey that is increasingly non-linear. We must ensure that we put consumers at the centre, create insights through analysis to understand the customer journey and thereby increase the relevance of our message. We are now increasing the capacity of our organisation and adding expertise and new tools together with marketing and sales,” says Sebastian Heimfors.
In 2021, the BioGaia brand was established in Finland and the UK and on 31 December its American partner, Everidis, was acquired.
In these markets and in Sweden and Japan, BioGaia now has direct responsibility for marketing.
“We are now testing content in various channels in our direct markets where it is easy to follow up and assess the response we receive. We want to support our partners and direct markets as part of the ongoing transformation and in this way we can develop much stronger tool boxes that can be adapted and used in their markets. For example, this could concern banners or short films to publish on YouTube or Instagram. We are building a strong, uniform BioGaia by supporting our partners with relevant marketing material for use in all platforms used by consumers,” says Linda Hägglund.
Same message in different ways

BioGaia increases its presence in key markets
During the year, BioGaia increased its presence in some markets to align with its omnichannel strategy to be relevant also to end consumers. Important steps were taken during the year to become even more accessible in highly attractive probiotics markets by establishing own companies and taking control of distribution in Finland and the UK, and by acquiring the American distribution partner Everidis.
BioGaia's markets

USA – BioGaia acquires its partner Everidis and strengthens position in the American market
2021 was another record year for BioGaia in the USA with a number of successful product launches. At the end of the year, BioGaia acquired its American partner of 13 years, Everidis, as a step to increase the pace of its initiative to directly reach consumers while continuing to market to the healthcare sector.
USA is a key market for BioGaia, where we have had a partnership since 2007 with Everidis, our exclusive distributor of products under BioGaia’s brand in the USA. Everidis has a well established omnichannel strategy and focuses on e-commerce rather than traditional pharmacy sales and marketing to consumers is almost exclusively digital. Doctors, dentists and other healthcare professionals are targeted by medical marketing. The omnichannel strategy has been highly successful and in keeping with the general digitalisation trend, which has now made the USA BioGaia’s largest market. Following the acquisition and support from BioGaia, Everidis will have more resources to further increase its pace, reach out directly to consumers and work with the healthcare sector.
With a customer value of USD 2 billion, the USA is the world’s largest probiotics market with excellent potential for continued growth. BioGaia’s close collaboration with Everidis in product development and building new product categories led to several successful product launches in 2021. Immune Boost was launched during the year, a range comprising drops for small children, tablets for children and capsules for adults. BioGaia has also noted growing interest for its dental tablets and launched Prodentis Kids tablets and Prodentis Baby Drops. All of the products were very warmly received by the market.
“BioGaia and Everidis have a tremendous collaboration and during the year we continued to build our brand, move closer to consumers and increase engagement through relevant content in all channels with excellent results.”
The idea of making the partnership permanent is brilliant, and we couldn’t be more excited to become part of the BioGaia family. Everidis’ talented team is building BioGaia into a powerful and trusted brand in the United States. With BioGaia, we will have access to resources and capabilities to help catapult growth and achieve the goal of becoming the leader of the science-driven probiotic category.



BioGaia at the forefront of research
A global collaboration
BioGaia has an extensive global research network and over the years has initiated partnerships with universities and hospitals, for example:
- ⦁ Swedish University of Agricultural Sciences in Uppsala
- ⦁ Karolinska Institute in Stockholm,
- ⦁ Sahlgrenska University Hospital in Gothenburg
- ⦁ Örebro University
- ⦁ Texas Children Hospital in the USA
- ⦁ The university hospitals in Bari and Turin in Italy
BioGaia aims to encourage the researchers its collaborates with to design studies and report results in a way that enables publication in reputable medical journals. The goal is also for all studies to have ethical approval and, regardless of the findings, should be published and public. The purpose is to increase transparency, share research findings and guarantee scientific relevance. BioGaia’s research network has published a total of 236 studies in medical journals over the years.
Research on the world’s most studied probiotics
BioGaias strains av Limosilactobacillus reuteri (L. reuteri) are some of the world’s most studied probiotics, especially when it comes to studies in young children. To date, 246 clinical studies with BioGaia’s human strains ofL. reuteri have been conducted, involving approximately 20,000 individuals of all ages (December 2021).
Over the years, studies have been carried out in the areas of:
- ⦁ Infantile colic and digestive health in children
- ⦁ Antibiotic-associated diarrhoea (AAD)
- ⦁ Acute diarrhoea
- ⦁ Gingivitis (inflammation of the gums)
- ⦁ Periodontal disease
- ⦁ General health
- ⦁ Helicobacter pylori (the gastric ulcer bacterium)
- ⦁ Osteopenia
Research 2021
When it’s possible, preventing rather than treating infectious or non-communicable diseases appears to be a valid strategy. This became even clearer during the Covid-19 pandemic outbreak. In 2021, BioGaia has, among other projects, initiated studies to examine the preventive role of L. reuteri DSM 17938 in conjunction with Vitamin D to prevent individuals from developing Covid-19.
Main milestones in BioGaia’s clinical research in 2021
⦁ A randomised placebo-controlled study on the potential protective effect of probiotics combined with vitamin D on Covid-19 was initiated during 2021.
⦁ A randomised controlled study published in April 2021 in Beneficial Microbes which showed that a combination of L. reuteri ATCC PTA 5289 och L. reuteri DSM 17938 may help reduce fever and sore throat pain in children under five years old with pharyngitis and tonsillitis. This study brings evidence on the importance of probiotics in helping our immune system to fight against common acute respiratory infections.
⦁ A randomised controlled study on subjects with diverticulitis showed a significant reduction of inflammatory markers associated with diverticulitis upon administration of Limosilactobacillus reuteri ATCC PTA 4659 compared to placebo.
At the end of 2021, BioGaia had more than 40 ongoing studies in various fields.

New collaborations through BioGaia Academy
BioGaia Academy
BioGaia Academy is the name of the education umbrella that covers BioGaia’s most important and most extensive external training. This major initiative started in 2017 when BioGaia developed training for paediatricians with the main goal of increasing knowledge of probiotics and encouraging global scientific collaboration. The programme has been developed in close cooperation with leading experts in the field who are also tutors for the course. The education mainly consists of digital online modules for self-study with some complementary webinars and face-to-face meetings to promote interaction and networking.
The main aim with BioGaia Academy is to raise awareness and knowledge of probiotics and their positive effects in a range of different areas where BioGaia has a strong scientific base. We also highlight studies and analyses by other groups to broaden the picture and knowledge about probiotics. The content and material used in the training is prepared together with our medical experts. The purpose is also to promote networking and communication among the participants and hence create more ambassadors for probiotics.
Our ambition is to have a limited number of participants per course to optimise learning for each participant. The candidates chosen to take part in our training are selected with the help our partners, and through BioGaia’s own internal contact network.
Value created by BioGaia Academy
Creating networks of researchers and people who are interested in a specific area contributes to valuable skills. Participants are from a variety of backgrounds and from every country in the world, and this enables us to gain new insights from the various experiences of participants, how products as used and what needs exist. Following the initial pilot course in paediatrics, which started in April 2018, additional training courses have been held. This included training in child health, training for dentists in oral health and a pilot course specifically for Chinese doctors, also in child health.
29 dentists from 14 countries took part in oral health training between May and November 2021. The training programmes were considered highly successful and, for example, a few of the participants came up with new ideas for clinical studies using probiotics. After the end of the programme, BioGaia held an additional webinar for the group and is planning more in 2022, as well as a face-to-face seminar (if the pandemic situation allows this).
The Expert Program Pediatrics China training programme was prepared together with BioGaia’s Chinese partner Chongqing Little Sunflower Health Industry Development Co. Ltd, in response to the need for probiotics training for Chinese doctors. The pilot course with six doctors began at the end of August 2021 and ended in mid-December 2021. BioGaia’s Chinese partner wants to start more programmes.
BioGaia’s goal has been to have a maximum of 20–30 doctors on each course. Some 35 doctors have graduated from the two courses within paediatrics we have held so far. From the round of training that began in 2018, both BioGaia and involved partners have continued to cooperate with more than 50% of participants who graduated. The participants’ knowledge level improved considerably during the first training programme that BioGaia Academy carried out, which indicates that there is still plenty to do.
First-class training in probiotics
BioGaia wants to offer first-class training in probiotics that is relevant and easily accessible for the target group. The ambition is to continue to develop BioGaia Academy’s training platform, both for external and internal target groups, by focusing more on the user’s perspective, creating variety with interactive modules and through short video lectures.
In addition to a partner programme in oral health, BioGaia is planning an expert programme for adult health (2023) and general training that focuses on child health.
Responsibility and contributions to sustainable development
Even if BioGaia focuses on the health of individuals through the company’s products, the term health has a broader meaning. We want our operationsto contribute positively to society as a whole.
BioGaia is striving to reduce the climate and environmental footprint and to ensure responsible behaviour throughout the value chain at the same time as we continue to be involved in societal issues that promote improved health.
Four focus areas for sustainability
The cornerstone of BioGaia’s sustainability work is healthy products based on research and clinical studies. One prerequisite is that these products are developed on the basis of sound operations where BioGaia’s networks and employees provide the foundation. To ensure that sustainability initiatives remain relevant and focused in those areas where BioGaia can make the most difference, a continuous dialogue is held with stakeholders about these issues.
Important sustainability events in 2021
-
The life-cycle assessment and tool to calculate environmental impacts from products were updated
-
BioGaia took part in a communication campaign on the subject of antibiotic resistance together with the national non-profit association SwedenBIO
-
The methods used to calculate the climate impact of operations were updated and further refined
-
SEK 3.6 million was donated to community engagement
-
The travel policy was updated to reduce both the cost and environmental impact of travel by personnel
-
100% green electricity throughout BioGaia’s operations
-
A new strategy for sustainable products and packaging was developed
-
Decision taken to introduce a Sustainability Committee in 2022
-
Anti-corruption training was prepared in a digital format to increase accessibility
Healthy products are key
By researching, developing and manufacturing products that also consider individual, environmental and social impacts, BioGaia strives to ensure products are healthy in every respect.
Research and product information
One of BioGaia’s strengths is the large number of well-executed independent clinical studies on our products and their health benefits over the past 30 years. With more than 236 published studies, BioGaia’s probiotic strains of Limosilactobacillus reuteri (L. reuteri) are some of the world’s most scientifically studied probiotics (see page 32).
Clear, transparent and easily accessible information about the research strengthens consumer confidence and is a cornerstone in BioGaia’s marketing. The company applies the International Scientific Association for Probiotics and Prebiotics (ISAPP) criteria for probiotic products, including how these should be labelled.
Stringent quality demands and constant improvements
Quality is one key aspect throughout the value chain – from ensuring that research on the product’s health benefits is reliable and there is a methodical production process, to the product maintaining its high quality after delivery to consumers. BioGaia checks every single batch that is produced against the applicable requirements. The compliance of contract manufacturers with the applicable quality requirements is also monitored through documented reviews and periodic audits. BioGaia has never needed to recall products already distributed to consumers. The company’s quality work also aims to identify potential improvements in documentation and production processes. For example, during 2020 the Protectis portfolio was declared allergen-free and in 2021 this was extended to cover more product families. During the year, BioGaia also optimised delivery flows leading to a reduction in transportation.
Priority to reduce impact from materials and ingredients
The result from the life-cycle assessment conducted in 2020 confirmed that a significant part of the environmental impact from BioGaia’s products stems from packaging and ingredients.
More sustainable packaging is one prioritised area and the additional life-cycle assessment conducted in 2021 provided valuable insights into the environmental impact of different packaging. The next stage is to develop packaging solutions that meet the requirements of retaining quality and sustainability for our products.

With respect to ingredients, the life-cycle assessment identified palm oil as the ingredient with the greatest impact on people, the climate and biodiversity. BioGaia is actively working to completely phase out palm oil. At the end of 2021, BioGaia could offer palm oil-free variants of the tablet product range in all markets and the roll-out of palm oil-free variants of drops (oil) is in progress. For the amount of palm oil used today, BioGaia buys credits from RSPO-certified independent smallholders in accordance with RSPO’s Book and Claim system.
BioGaia’s goal is to have completely phased out products that contain palm oil by 2025. As part of this work, BioGaia has recruited one person during the year to work full time with this phase out.
Our core values
Innovation
We strive for breakthrough solutions that make a difference in peoples’ lives.
Collaboration
Trust, helpfulness and curiosity define our way of working.
Passion
We are committed and put our hearts into everything we do.
